(See what I did there? That's clever and you know it, even if it is overused)
Getting back to the roots of this blog, it's time I talk about something that made me look it up. The current synthesis our group is working on, a molecule with no name yet, is described as a "Lignan". It is also described as a number of other things as well, but that's going to require more space than I have to cover it all. But, in an effort to understand more about this molecule, I did have to look up "lignans", and since that's the case, I have to blog about it as well.
Lignans are a group of chemical compounds that are mostly found in plants. They generally take the form of two aromatic ring, usually phenolic, connected by a chain that, on some level, can be considered symmetric. An example of a lignan is Enterodiol.
They are a major subgroup of the estrogen-like phytoestrogens and can act as an anti-oxidant, making them pharmacologically interesting.
What you don't want to do is get these confused with lignins, which are much larger compounds commonly found in wood. (As a side note, most of the artificial vanilla made today comes from lignins as a by-product of the wood-pulping industry.)
However, both lignans and lignins can be formed by a precusor called a monolignol. These most commonly take the form of substituted cinnamyl alcohols. In fact, the first step of our groups synthesis is forming a substituted cinnamyl alcohol.
If you wanted to go hunting for lignans, you'll most likely find them in sesame seeds and flaxseeds. Mammals can modify some lignans ingested and create their own too. New lignans are being discovered regularly, too. Li, Zhenxing et al. published a paper in Chemistry of Natural compounds describing two lignans found in the roots of the Chinese Fir back in May of this year.
Sorry this post is not as interesting, but its a great way to keep learning and be able to better converse with your peers.
-Woodward
Thursday, August 29, 2013
What the R.F. Heck is: a Lignan?
Labels:
cinnamyl alcohol,
Heck,
Highlights,
lignan,
lignin,
monolignol
Tuesday, August 27, 2013
Chemophobia - and How to Deal With It
In the post Keeping up with the Jonses I talked about trends in Organic Chemistry, discussing things like 'green chemistry' and calcium catalysts. On a more meta level, there are trends about the discussion of chemistry, on blogs, podcasts, etc. In case you haven't been keeping up-to-date, Chemophobia (if your Latin's a bit rusty, "the fear of chemicals") is the go to topic ('fraud' also happens to be kind of big too). I figure I should throw in my two cents (0.0128 pounds sterling at current exchange rates).
In popular culture, it is fashionable to be afraid of chemicals. I'm not saying that people are running in fear, just that they tend to avoid it when possible. Natural diets, home cures, organic foods, and the like are all the rage and promoted by high profile individuals. There's nothing wrong with a person's right to choose how they live their lives, but it brings about a fear of anything that can be labeled "unnatural". And as much as we despise it, the word "chemical" has been branded "unnatural".
Can't really blame the people, there have been some unfortunate run-ins with "chemicals" in the past; DDT and Agent Orange come to mind. It is unfortunate the bad consequences of these chemicals have been placed on good chemicals as well. Pesticides and prescription drugs all get a bad wrap. Public reaction, however, has become over-reactionary. Walk into a your local big box store and you invariably find soap, cleaners, and the like labelled as "chemical-free". Never mind that it still has chemicals like water in it. Never mind that the formula hasn't changed in 50 years. People will appreciate and gravitate towards these products because of the pejorative use of the word chemical. And this sort of use of the word 'chemical' will continue until chemophobia starts being less prevalent. And how do we that?
By making Chemistry more accessible. The argument has been made that Chemistry is the least accessible science. Astronomy has lots to look at, physics makes roller coasters go, biology is squishy but still understandable. Chemistry doesn't have any sort of equivalent. It is inaccessible to the Average Joe. One of the reasons I started this blog is because I felt much of the chemistry going on today was inaccessible to an undergrad as well. We need to find ways in which chemistry can be more easily understood by the population in general, not just by professors and post-docs. This will be an uphill battle, and there is no easy solution, but if we start now, by blogging, talking with friends, using social media, and the sort, perhaps we can make chemistry a more friendly field for the next generation of scientists.
-Woodward
A great article at The Collapsed Wavefunction talks about not "punching down" chemophobia.
See Arr Oh wrote a great article highlighting the pervasiveness of chemophobia at Just Like Cooking.
Here's a graphic that shows how everyday chemicals can have scary sounding names.
Edit - Perhaps xkcd has the best way to deal with chemophobics
In popular culture, it is fashionable to be afraid of chemicals. I'm not saying that people are running in fear, just that they tend to avoid it when possible. Natural diets, home cures, organic foods, and the like are all the rage and promoted by high profile individuals. There's nothing wrong with a person's right to choose how they live their lives, but it brings about a fear of anything that can be labeled "unnatural". And as much as we despise it, the word "chemical" has been branded "unnatural".
Can't really blame the people, there have been some unfortunate run-ins with "chemicals" in the past; DDT and Agent Orange come to mind. It is unfortunate the bad consequences of these chemicals have been placed on good chemicals as well. Pesticides and prescription drugs all get a bad wrap. Public reaction, however, has become over-reactionary. Walk into a your local big box store and you invariably find soap, cleaners, and the like labelled as "chemical-free". Never mind that it still has chemicals like water in it. Never mind that the formula hasn't changed in 50 years. People will appreciate and gravitate towards these products because of the pejorative use of the word chemical. And this sort of use of the word 'chemical' will continue until chemophobia starts being less prevalent. And how do we that?
By making Chemistry more accessible. The argument has been made that Chemistry is the least accessible science. Astronomy has lots to look at, physics makes roller coasters go, biology is squishy but still understandable. Chemistry doesn't have any sort of equivalent. It is inaccessible to the Average Joe. One of the reasons I started this blog is because I felt much of the chemistry going on today was inaccessible to an undergrad as well. We need to find ways in which chemistry can be more easily understood by the population in general, not just by professors and post-docs. This will be an uphill battle, and there is no easy solution, but if we start now, by blogging, talking with friends, using social media, and the sort, perhaps we can make chemistry a more friendly field for the next generation of scientists.
-Woodward
A great article at The Collapsed Wavefunction talks about not "punching down" chemophobia.
See Arr Oh wrote a great article highlighting the pervasiveness of chemophobia at Just Like Cooking.
Here's a graphic that shows how everyday chemicals can have scary sounding names.
Edit - Perhaps xkcd has the best way to deal with chemophobics
Tuesday, August 20, 2013
Class Notes 1 - Hammond's Postulate
I should really call it the Hammond-Leffler postulate, but popularity can be influential in chemistry I suppose.
Class hasn't actually started yet, I have a couple of more weeks before I really have to worry about that. But my (future) Advanced Organic Chemistry professor advised that we start studying a little early so we are not caught off-guard. One of his biggest concerns is that students will be unprepared by the Hammond Postulate. As a refresher for some of you out there, Hammond's postulate is that the transition state of a reaction will resemble the species closest to it in energy. This makes sense, since most of the time when drawing transition state diagrams, the 'peak' is close on the reaction coordinate to whatever is higher in energy. If the transition state is close in energy to the higher energy species, you can approximate this transition state as being the higher energy species. This picture, courtesy of Master Organic Chemistry, summarizes it nicely:
As an example, an alkene being converted into a carbocation will have a transition state that resembles the higher energy carbocation.
For me, this seems intuitive. Heck, I'm fairly certain that we learned this in first semester OChem. Why then is our professor concerned about us not being able to understand it? From asking around, it seems that applying the postulate to actual reactions is where things are going to be tricky.
What do you think? Am I missing something here that makes this more complicated than I understand it to be? Am I going to get my butt kicked this semester? If this is all there is to it, I'm hoping that this is going to be the most difficult concept we learn. Oh, who am I kidding.
-Woodward
Class hasn't actually started yet, I have a couple of more weeks before I really have to worry about that. But my (future) Advanced Organic Chemistry professor advised that we start studying a little early so we are not caught off-guard. One of his biggest concerns is that students will be unprepared by the Hammond Postulate. As a refresher for some of you out there, Hammond's postulate is that the transition state of a reaction will resemble the species closest to it in energy. This makes sense, since most of the time when drawing transition state diagrams, the 'peak' is close on the reaction coordinate to whatever is higher in energy. If the transition state is close in energy to the higher energy species, you can approximate this transition state as being the higher energy species. This picture, courtesy of Master Organic Chemistry, summarizes it nicely:
As an example, an alkene being converted into a carbocation will have a transition state that resembles the higher energy carbocation.
For me, this seems intuitive. Heck, I'm fairly certain that we learned this in first semester OChem. Why then is our professor concerned about us not being able to understand it? From asking around, it seems that applying the postulate to actual reactions is where things are going to be tricky.
What do you think? Am I missing something here that makes this more complicated than I understand it to be? Am I going to get my butt kicked this semester? If this is all there is to it, I'm hoping that this is going to be the most difficult concept we learn. Oh, who am I kidding.
-Woodward
Labels:
class notes,
Hammond,
postulate,
transition states
Friday, August 16, 2013
Keeping Up with the Joneses
When being taught undergrad organic chemistry, one tends to consider it as a pure, unadulterated discipline. Once you start going over journals, reading blogs, and keeping up with the news, it becomes apparent that there are politics, popularity contests, public demand, and ever-shifting trends that influence the state of the science. Since I and many other undergrads are soon to be entering the game, I think it prudent to cover a few of the current trends to help us stay ahead of the curve.
One of the biggest trends right now is 'Green' Chemistry. You know, the kind of chemistry that's good for the environment and what not. This strikes me as a little odd, because this trend arises not from the needs of researchers to improve experiments, but from politics and moral convictions. I'm not saying that it is wrong for us to move in this direction (in fact I support it), but merely note how interesting it is that such a trend is not influenced by reactions in the lab or hypothesis on a chalk board. A good example of the rise in organic chemistry is an editorial over at ACS about replacing solvents with 'green' counterparts.
Some other trends are listed on a Wikipedia page and include chiral syntheses, flow chemistry, and microwave chemistry. For those of you who are unaware, microwave chemistry is literally what it sounds like. BRSM has a great post discussing microwave chemistry, you should check it out. The Wikipedia section on trends was last updated in 2008, so expect some of these to be out of style and others to have taken their place.
What's next on the organic chemistry trends list? See Arr Oh over at justlikecooking.blogspot.com has a recent article about a rise in calcium catalysts. It's not quite a wide-spread trend yet, but it could be the next big thing. While your there check out some of the other recent trends linked to in the article, like the 'Gold Rush'.
Your homework is to look for themes, tendencies, and trends in the articles you read. That way you'll be on top of your game and ready to play.
-Woodward
One of the biggest trends right now is 'Green' Chemistry. You know, the kind of chemistry that's good for the environment and what not. This strikes me as a little odd, because this trend arises not from the needs of researchers to improve experiments, but from politics and moral convictions. I'm not saying that it is wrong for us to move in this direction (in fact I support it), but merely note how interesting it is that such a trend is not influenced by reactions in the lab or hypothesis on a chalk board. A good example of the rise in organic chemistry is an editorial over at ACS about replacing solvents with 'green' counterparts.
Some other trends are listed on a Wikipedia page and include chiral syntheses, flow chemistry, and microwave chemistry. For those of you who are unaware, microwave chemistry is literally what it sounds like. BRSM has a great post discussing microwave chemistry, you should check it out. The Wikipedia section on trends was last updated in 2008, so expect some of these to be out of style and others to have taken their place.
What's next on the organic chemistry trends list? See Arr Oh over at justlikecooking.blogspot.com has a recent article about a rise in calcium catalysts. It's not quite a wide-spread trend yet, but it could be the next big thing. While your there check out some of the other recent trends linked to in the article, like the 'Gold Rush'.
Your homework is to look for themes, tendencies, and trends in the articles you read. That way you'll be on top of your game and ready to play.
-Woodward
Labels:
calcium catalysts,
Current Events,
green,
microwave,
trends
Wednesday, August 14, 2013
What Makes a Synthesis Elegant?
This is a topic that I've been wondering about for some time. I'll often hear someone (or an abstract if they're not-so-humble) describe a synthesis as being elegant. It's definitely not a word that's thrown around the scientific community often, as we tend to be more concerned with results, data, and other unemotional things. However there tends to be a push towards finding 'elegant' solutions to syntheses, and more often than not an elegant solution will be prized above one that was published earlier.
So what, exactly, makes a synthesis elegant? Asking around today brought me a variety of answers. Most came in two parts. First they highlighted certain types of reactions and solutions, citing stereoselectivity, strategic bonding, and the like. Then, most said something along the lines of, "an elegant synthesis should make you feel like you are looking at a piece of art." Given that last sentence and that all 'elegant' solutions are different, quantifying this would be impossible. But there's no harm in trying!
After talking it over, looking up syntheses, and musing to myself, here's how you go about making an elegant synthesis:
So what, exactly, makes a synthesis elegant? Asking around today brought me a variety of answers. Most came in two parts. First they highlighted certain types of reactions and solutions, citing stereoselectivity, strategic bonding, and the like. Then, most said something along the lines of, "an elegant synthesis should make you feel like you are looking at a piece of art." Given that last sentence and that all 'elegant' solutions are different, quantifying this would be impossible. But there's no harm in trying!
After talking it over, looking up syntheses, and musing to myself, here's how you go about making an elegant synthesis:
- It must be short. Like 10-20 steps total short.
- It must rapidly generate complexity. Taking 5 steps to protect/deprotect isn't helping you.
- It must have some novel or unique approach. Not necessarily complex reactions, just combining more basic ones in a way no one has thought of before is fine.
- If it's a newly synthesized compound, you get bonus points.
At the end of the day, there are going to be some reactions that fit these rules that won't be considered elegant. And elegant solutions are not always going to follow this approach. When it comes down to it, when you see an elegant synthesis, you will be able to appreciate it. And I suppose that comes with experience. I imagine its like watching a Grand-master play chess. To the uninitiated, it may be a convoluted mess with no real pattern until suddenly, check-mate. To an experienced player, however, each move carries significance, strategy, and meaning. So that as you watch, it transcends being a game, and becomes a work of art.
-Woodward
Some thoughts from twitter
@NatureChemistry @WoodwardRB - But seriously: "Elegance" utilizes strategic bonds and interesting reactions synergistically (1/2)
— See Arr Oh (@SeeArrOh) August 14, 2013
@NatureChemistry @WoodwardRB - "Elegance" causes a deep, fundamental emotional reaction to the work as a whole, like a contented sigh (2/2)
— See Arr Oh (@SeeArrOh) August 14, 2013
@WoodwardRB A synthesis that makes me feel the design of the route did all the work. (1/2)
— Stephen Davey (@stephengdavey) August 14, 2013
@WoodwardRB So that at each step it seems like the molecule is just being encouraged rather than forced to do something.(2/2)
— Stephen Davey (@stephengdavey) August 14, 2013
@WoodwardRB Early generation of one asymmetric center, and then diastereoselective rxns to generate all others, Ex: http://t.co/gNQs72uy9R
— Thomas GD (@ThomasGaleandro) August 14, 2013
@WoodwardRB More generally : its shortness, with rapid generation of complexity. Bonus if it uses wisely catalysts ;)
— Thomas GD (@ThomasGaleandro) August 14, 2013
@WoodwardRB I would say synthesis is elegant if there is a beautiful usage of rearrangement that cuts the sequence down for several stepsLeave feedback on what you think makes a synthesis 'elegant' or leave an example of an example of an elegant synthesis.
— Xydja (@Xydja) August 14, 2013
Labels:
chess,
elegant synthesis,
specials,
syntheses,
twitter
Monday, August 12, 2013
An Undergrad's Take on the Controversy in 'Organometallics'
If there was ever proof that there is more to the world of chemistry than just arrow pushing...
In case you haven't heard, last week ChemBark reported on a disturbing note in the SI portion of an ASAP article in the journal Organometallics. The note, apparently from the principal investigator to the first author, states that they need an NMR in a certain location, and that she should "just make up an elemental analysis..." Much has been said already on this potential fraud, and ChemBark is doing an excellent job at chronicling events as they unfold, so I won't go into the details. However, I feel that this blog, created by the undergrad for the undergrad, allows for a fresh perspective on the situation.
If this note is legitimately calling for researchers to make up data, than it is morally, ethically, and professionally wrong. However, I would not be surprised if many researchers have been tempted by this easy route. Lets face it, the supporting information hardly ever gets read. This article provides the perfect example: the note made it through three peer reviews without mention and was on the site for almost a month before anyone pointed it out. If this awkward, out-of-place note made it that far, surely there are people who've considered - and some who have - burying erroneous, falsified, but camouflaged data deep in the SI. Especially when there's a deadline to reach.
This doesn't make what was done inexcusable, but it does highlight what is likely to be found a growing problem, a cancer, in the chemistry community. We must hold ourselves to a higher standard if we want the scientific community to continue being a paragon of honesty, thoroughness, and truth.
-Woodward
In case you haven't heard, last week ChemBark reported on a disturbing note in the SI portion of an ASAP article in the journal Organometallics. The note, apparently from the principal investigator to the first author, states that they need an NMR in a certain location, and that she should "just make up an elemental analysis..." Much has been said already on this potential fraud, and ChemBark is doing an excellent job at chronicling events as they unfold, so I won't go into the details. However, I feel that this blog, created by the undergrad for the undergrad, allows for a fresh perspective on the situation.
If this note is legitimately calling for researchers to make up data, than it is morally, ethically, and professionally wrong. However, I would not be surprised if many researchers have been tempted by this easy route. Lets face it, the supporting information hardly ever gets read. This article provides the perfect example: the note made it through three peer reviews without mention and was on the site for almost a month before anyone pointed it out. If this awkward, out-of-place note made it that far, surely there are people who've considered - and some who have - burying erroneous, falsified, but camouflaged data deep in the SI. Especially when there's a deadline to reach.
This doesn't make what was done inexcusable, but it does highlight what is likely to be found a growing problem, a cancer, in the chemistry community. We must hold ourselves to a higher standard if we want the scientific community to continue being a paragon of honesty, thoroughness, and truth.
-Woodward
Labels:
chembark,
controversy,
Current Events,
organometallics,
standards
Thursday, August 8, 2013
Notes From Group Meeting - 1
Today was our group meeting, and since it is also the end of the summer semester, the last one until Fall semester starts. So no more of these for a couple of weeks. That being said, there were a few things I learned today that I thought worth sharing.
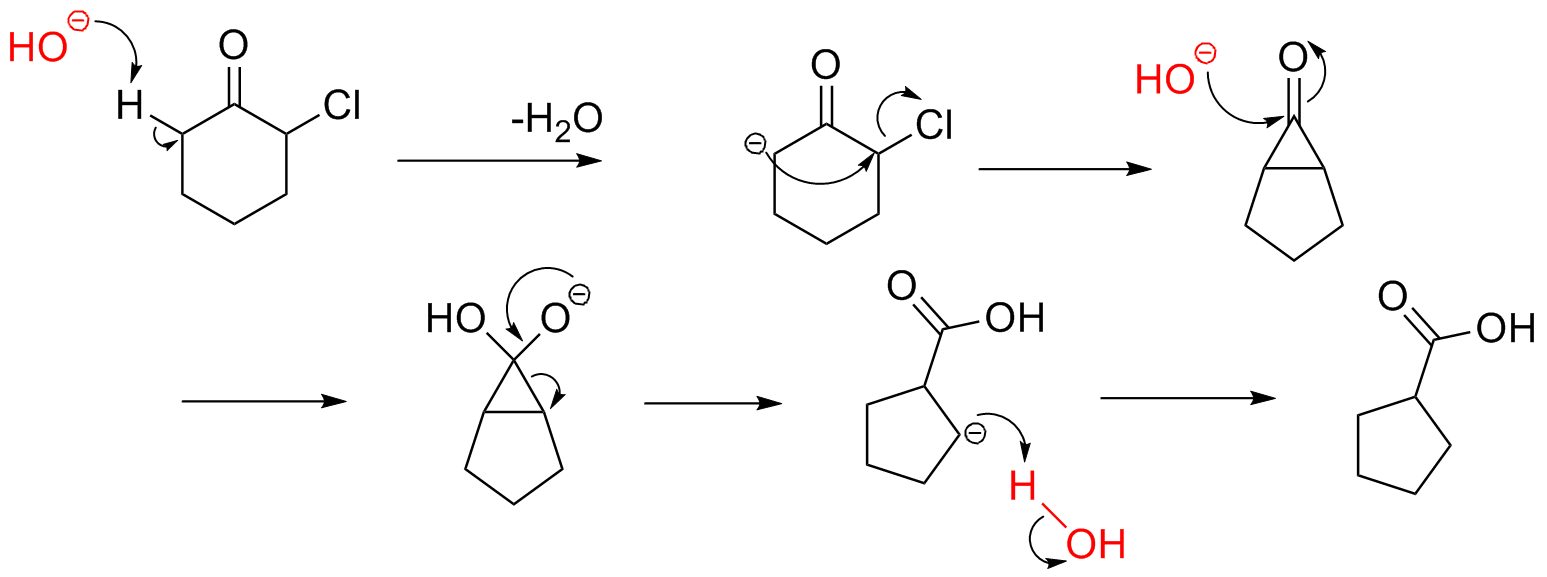
First up is from one of our problem sets. One professor, having not seen the problem before, said, "Ah yes, a Favorskii rearrangement!" I, of course, had never heard of it. Turns out it was more complex than I expected and thought you ought to know about it.
Alexey Yevgrafovich Favorsky was a Russian chemist back at the turn of the century (the 19th to 20th turn, not the most recent one). He was an accomplished scientist, and besides this rearrangement and one other reaction, his most notable achievement was receiving the Stalin award for improving synthetic rubber.
The Favorskii rearrangement is most commonly used to constrict ring, generally from a six member ring to a five member one. The general mechanism is as follows:
First up is from one of our problem sets. One professor, having not seen the problem before, said, "Ah yes, a Favorskii rearrangement!" I, of course, had never heard of it. Turns out it was more complex than I expected and thought you ought to know about it.
Alexey Yevgrafovich Favorsky was a Russian chemist back at the turn of the century (the 19th to 20th turn, not the most recent one). He was an accomplished scientist, and besides this rearrangement and one other reaction, his most notable achievement was receiving the Stalin award for improving synthetic rubber.
The Favorskii rearrangement is most commonly used to constrict ring, generally from a six member ring to a five member one. The general mechanism is as follows:
With that in mind, can you figure this out? Click the picture for the answer.
Edit: Spelled "Favorskii" wrong
Wednesday, August 7, 2013
R. B. Woodward
 |
| The Man, the Myth, the Legend |
Why is Woodward so important? He practically created the field of organic synthesis. Doing most of his work in the 1940's and 50's, he has been described as "the preeminent organic chemist of the twentieth century," and no one seems to disagree. He synthesized quinine, cortisol, cholesterol, chlorophyll, and many more. He is the reason many chemists look for elegance in their syntheses. (The subject of what makes a synthesis "elegant" is sure to be the subject of a future post.) He published over 85 papers, some having to be published after his death because of the blindingly fast pace he set for himself. He won the 1965 Nobel Prize in Chemistry, not for any one molecule, but for his "synthesis of many complex organic molecules." Some say he should have won more. Because of his great contributions to the field of organic chemistry, I chose as my moniker the name of "Woodward". Should I not aspire to great things?
Read more on Woodward here.
-Woodward
Labels:
chemist,
Highlights,
nobel prize,
people,
synthesis,
Woodward
More Than Arrow Pusing
I'm an undergrad student living in the US and I'm staring graduation in the face. I've taken almost every chemistry course, have done my time in two separate labs on campus, and am attempting to keep up-to-date on the field's current findings. In an attempt to become more scientifically literate, I've started reading blogs and perusing textbooks. In that attempt, I've come to find one thing: Synthetic Chemistry is more than arrow pushing. What do I mean by that? I mean that I can work through schemes and show the correct bonding mechanism. I can read NMR's and Mass Spec readouts. I can follow procedures and get good yields on my reactions. However, there are subtexts and nuances that I seem to miss more often than not. In group meetings, two professors will joke with each other on how difficult a certain compound might have been to work with, while I sit there never even knowing that it was so difficult. Often times a paper will be published and someone will say: "Oh, that group is always doing good work" or "So-and-so published? I wonder how mundane it will be." There may be lists of compounds and working conditions listed for a given reaction, and a professor know immediately which ones will be the cheapest and/or most effective. There are no classes that teach you these things, and I realize that much of this is experience talking. But there's nothing wrong with a jump-start, right?
So I will put on this blog the new things that I've learned, come-across, or stumbled upon while reading papers and blogs and sitting in group meetings. I hope this is the longest post I make. I also hope that this inspires other undergrads like me to continue learning, even when its outside the bounds of classes.
-Woodward
So I will put on this blog the new things that I've learned, come-across, or stumbled upon while reading papers and blogs and sitting in group meetings. I hope this is the longest post I make. I also hope that this inspires other undergrads like me to continue learning, even when its outside the bounds of classes.
-Woodward
Subscribe to:
Posts (Atom)